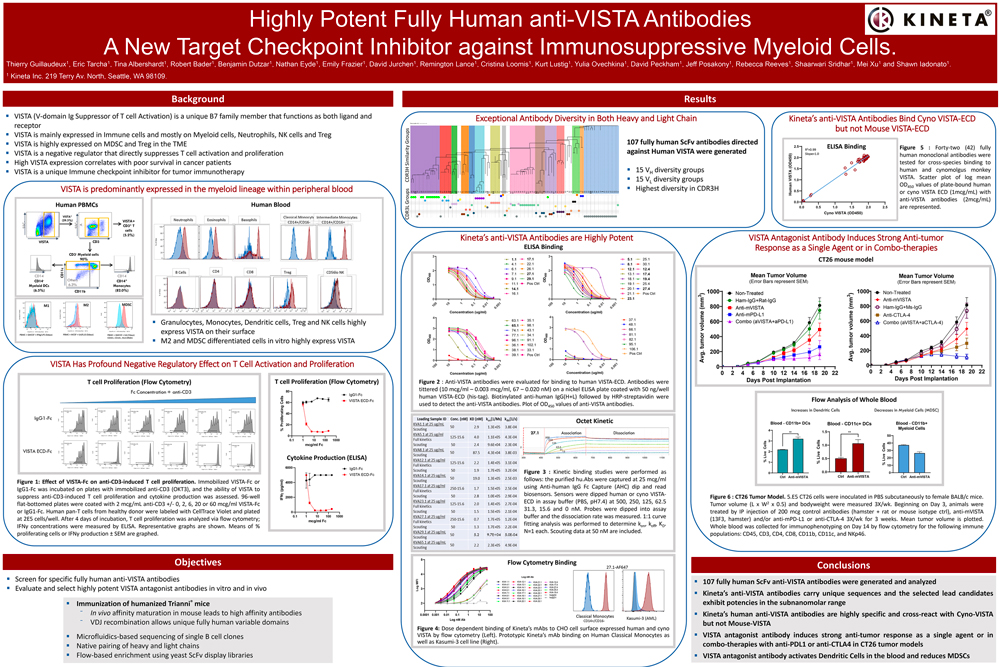
AddressingImmunosuppression in the TME

Addressing immunosuppression in the TME

KVA12123
Anti-VISTA mAb
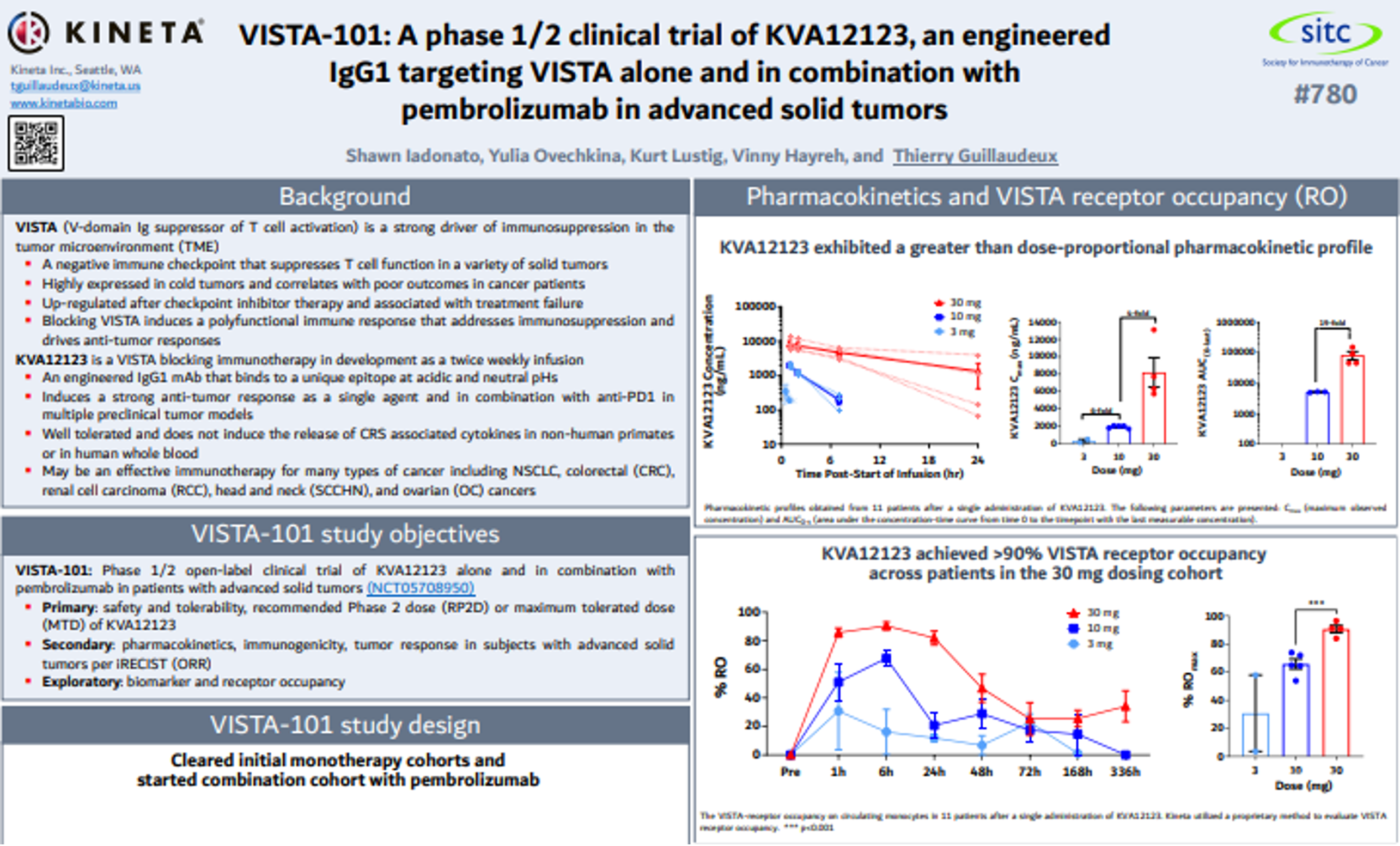
KVA12123 is designed to be a differentiated VISTA blocking immunotherapy to address the problem of immunosuppression in the tumor microenvironment (TME). KVA12123 is a VISTA blocking immunotherapy in development as an infusion dosed every two weeks.
Through the combination of unique epitope binding and an optimized IgG1 Fc region, KVA12123 demonstrates strong monotherapy tumor growth inhibition in preclinical models without evidence of cytokine release syndrome (CRS) in clinical trial participants. KVA12123 also exhibits an excellent safety profile in initial clinical trial monotherapy cohorts.
KVA12123 has been shown to de-risk the VISTA target and provides a novel approach to address the problem of immuno-suppression in the TME with a mechanism of action that is differentiated and complementary to T cell focused therapies.
The drug is being evaluated in an ongoing Phase 1/2 clinical trial as a monotherapy and in combination with pembrolizumab in patients with advanced solid tumors.


Target
VISTA (V-domain Ig suppressor of T cell activation) is a negative immune checkpoint that suppresses T cell function in a variety of solid tumors. High VISTA expression in tumor correlates with poor survival in cancer patients and has been associated with a lack of response to other immune checkpoint inhibitors. Blocking VISTA induces an efficient polyfunctional immune response to address immunosuppression and drives anti-tumor responses.
VISTA is a novel immuno-oncology target due to its unique expression and activity. First, high VISTA expression on immunosuppressive myeloid cells (tumor associated macrophages and myeloid-derived suppressor cells (MDSCs)) is consistent across tumor types, making it a relevant target across multiple types of cancer. Re-programmed myeloid cells can drive tumor inflammation. VISTA-blockade decreases immune suppression and provides single agent tumor growth inhibition and also improves efficacy of T cell focused therapies like anti-PD(L)1 and anti-CTLA4.
Second, blocking VISTA induces activation of dendritic cells and natural killer (“NK”) cells and ultimately proliferation and infiltration of T cells into the tumor. The combination of myeloid, NK and T cell responses can reverse immunosuppression and drive anti-tumor activity. While many immuno-oncology targets address either T cell or myeloid functions, VISTA has the potential to regulate both.

There is a strong clinical rationale for targeting VISTA with an antibody immunotherapy. The innate immune target VISTA is highly expressed in many types of cancer, including non-small cell lung cancer (NSCLC), colorectal cancer, ovarian cancer, renal cell carcinoma, and head and neck squamous cell carcinoma.

VISTA-101Phase 1/2 Clinical Trial
VISTA-101 is a first-in-human, Phase 1/2, open-label, multicenter, dose escalation, and dose expansion study designed to evaluate the safety, tolerability, pharmacokinetics (PK), immunogenicity, and tumor response of the investigational drug KVA12123 alone as a monotherapy and in combination with pembrolizumab in adults with relapsed or refractory advanced solid tumors.


Collaboration with Merck
Kineta entered into a clinical trial collaboration and supply agreement with Merck (known as MSD outside the U.S. and Canada). Under this collaboration, Kineta will evaluate the safety, tolerability, pharmacokinetics and anti-tumor activity of KVA12123 (formerly KVA12.1), its novel anti-VISTA monoclonal antibody, alone and in combination with KEYTRUDA® (pembrolizumab), Merck’s anti-PD-1 therapy, in patients with advanced solid tumors.

Publications
Thierra Guillaudeux, PhD, Chief Scientific Officer, Kineta Inc American Association of Cancer Research Annual Meeting | April 8, 2024
Thierry Guillaudeux; Shaarwari Sridhar; Emily Frazier; Yulia Ovechkina; Shawn Iadonato AACR Journal: Blood Cancer Discovery | March 4, 2024
Shawn Iadonato, Yulia Ovechkina, Kurt Lustig, Jessica Cross, Nathan Eyde, Emily Frazier, Neda Kabi, Chen Katz, Remington Lance, David Peckham, Shaarwari Sridhar, Carla Talbaux, Isabelle Tihista, Mei Xu, Thierry Guillaudeux* Frontiers in Immunology | December 12, 2023
Thierry Guillaudeux, Shaarwari Sridhar, Emily Frazier, Yulia Ovechkina, Shawn Iadonato Blood - American Society of Hematology | November 28, 2023
Thierry Guillaudeux, Phd, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2023 | November 3, 2023
Randolph J. Noelle, J. Louise Lines, Lionel D. Lewis, Robert E. Martell, Thierry Guillaudeux, Sam W. Lee, Kathleen M. Mahoney, Matthew D. Vesely, Jerome Boyd-Kirkup, Dhanya K. Nambiar and Andrew M. Scott Frontiers in Oncology | September 15, 2023
Thierry Guillaudeux, PhD, Chief Scientific Officer American Association for Cancer Research (AACR) Annual Meeting 2023 | April 16, 2023
Thierry Guillaudeux, PhD, Chief Scientific Officer Antibody Engineering & Therapeutics Conference | December 7, 2022
Thierry Guillaudeux, PhD, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2022 | November, 2022

KVA 12.1 a novel fully human anti-VISTA antibody to treat cancer patients with advanced solid tumors
Shaarwari Sridhar, Scientist at Kineta Tumor Myeloid-Directed Therapies Summit 2022 | June 14, 2022
Thierry Guillaudeux, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2021 | November, 2021
Thierry Guillaudeux, PhD, Chief Scientific Officer American Association for Cancer Research (AACR) Annual Meeting 2021 | April, 2021
Thierry Guillaudeux, PhD, Chief Scientific Officer European Society for Medical Oncology (ESMO) Virtual Congress 2020 | December 9, 2020
Thierry Guillaudeux, PhD, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 35th Anniversary Annual Meeting | November 12, 2020
Thierry Guillaudeux, PhD, Chief Scientific Officer American Association for Cancer Research (AACR) Virtual Special Conference: Tumor Immunology and Immunotherapy | October 19-20, 2020
Thierra Guillaudeux, PhD, Chief Scientific Officer, Kineta Inc American Association of Cancer Research Annual Meeting | April 8, 2024
Thierry Guillaudeux; Shaarwari Sridhar; Emily Frazier; Yulia Ovechkina; Shawn Iadonato AACR Journal: Blood Cancer Discovery | March 4, 2024
Shawn Iadonato, Yulia Ovechkina, Kurt Lustig, Jessica Cross, Nathan Eyde, Emily Frazier, Neda Kabi, Chen Katz, Remington Lance, David Peckham, Shaarwari Sridhar, Carla Talbaux, Isabelle Tihista, Mei Xu, Thierry Guillaudeux* Frontiers in Immunology | December 12, 2023
Thierry Guillaudeux, Shaarwari Sridhar, Emily Frazier, Yulia Ovechkina, Shawn Iadonato Blood - American Society of Hematology | November 28, 2023
Thierry Guillaudeux, Phd, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2023 | November 3, 2023
Randolph J. Noelle, J. Louise Lines, Lionel D. Lewis, Robert E. Martell, Thierry Guillaudeux, Sam W. Lee, Kathleen M. Mahoney, Matthew D. Vesely, Jerome Boyd-Kirkup, Dhanya K. Nambiar and Andrew M. Scott Frontiers in Oncology | September 15, 2023
Thierry Guillaudeux, PhD, Chief Scientific Officer American Association for Cancer Research (AACR) Annual Meeting 2023 | April 16, 2023
Thierry Guillaudeux, PhD, Chief Scientific Officer Antibody Engineering & Therapeutics Conference | December 7, 2022
Thierry Guillaudeux, PhD, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2022 | November, 2022

KVA 12.1 a novel fully human anti-VISTA antibody to treat cancer patients with advanced solid tumors
Shaarwari Sridhar, Scientist at Kineta Tumor Myeloid-Directed Therapies Summit 2022 | June 14, 2022
Thierry Guillaudeux, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2021 | November, 2021
Thierry Guillaudeux, PhD, Chief Scientific Officer American Association for Cancer Research (AACR) Annual Meeting 2021 | April, 2021
Thierry Guillaudeux, PhD, Chief Scientific Officer European Society for Medical Oncology (ESMO) Virtual Congress 2020 | December 9, 2020
Thierry Guillaudeux, PhD, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 35th Anniversary Annual Meeting | November 12, 2020
Thierry Guillaudeux, PhD, Chief Scientific Officer American Association for Cancer Research (AACR) Virtual Special Conference: Tumor Immunology and Immunotherapy | October 19-20, 2020
References:
1. Rebecca L. Siegel et al.; Cancer statistics, 2020; ACS Journals; First published: 08 January 2020 https://doi.org/10.3322/caac.21660
2. GlobalData epidemiology forecasts for NSCLC, CRC, OC