
Kineta Announces FDA Acceptance of Investigational New Drug (IND) Application for KVA12123 for the Treatment of Advanced Solid Tumors
Seattle, WA – (November 15, 2022) Kineta, Inc. (“Kineta” or the “Company”), a clinical-stage biotechnology company focused on developing next-generation immunotherapies that address cancer immune resi ...

Kineta Announces Effectiveness of Registration Statement on Form S-4 in Connection with Proposed Reverse Merger with Yumanity Therapeutics (YMTX)
Special Meeting of stockholders to approve asset sale and merger to be held on December 13, 2022 BOSTON, Nov. 10, 2022 -- Yumanity Therapeutics, Inc. ("Yumanity" or the "Company") (Nasdaq: YMTX) ...
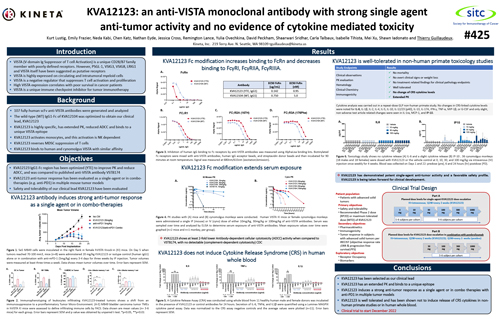
KVA12123: an anti-VISTA monoclonal antibody with strong single agent anti-tumor activity and no evidence of cytokine mediated toxicity
Thierry Guillaudeux, PhD, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2022 | November, 2022

Kineta to Present at Pre-clinical Data on VISTA and CD27 Programs at SITC 2022 Annual Meeting
Seattle, WA — (October 27, 2022) Kineta, Inc. (“Kineta” or the “Company”), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology, announced today that ...

Kineta Announces Clinical Collaboration with Merck to Evaluate KVA12123 in Combination with KEYTRUDA® (pembrolizumab) in Cancer Patients with Advanced Solid Tumors
Kineta’s VISTA blocking immunotherapy KVA12123 (formerly referred to as KVA12.1) to be evaluated in patients with advanced solid tumors Clinical trial anticipated to begin in late 2022 Seattle, ...

Kineta to Present Anti-CD27 Agonist Preclinical Program at AACR Conference on Tumor Immunology and Immunotherapy
Seattle, WA – (October 12, 2022) Kineta, Inc. (“Kineta” or the “Company”), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology, announced today that ...

Kineta Announces Participation in Upcoming October 2022 Investor Conferences
Seattle, WA – (October 10, 2022) Kineta, Inc. (“Kineta” or the “Company”), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology, announced today that ...

Kineta CSO Thierry Guillaudeux Invited to Participate at the 2nd Annual VISTA Virtual Symposium
Seattle, WA — (September 14, 2022) Kineta, Inc. (“Kineta” or the “Company”), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology, announced today tha ...

Kineta Announces Participation in the H.C. Wainwright 24th Annual Global Healthcare Conference
Seattle, WA — (September 12, 2022) Kineta, Inc. (“Kineta” or the “Company”), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology, announced today th ...
