
VISTA a Potential New Immuno-Oncology Target in Acute Myeloid Leukemia
Thierry Guillaudeux, Shaarwari Sridhar, Emily Frazier, Yulia Ovechkina, Shawn Iadonato Blood - American Society of Hematology | November 28, 2023

Kineta to Host KOL Event to Review New Data from VISTA-101 Clinical Trial of KVA12123 in Patients with Advanced Solid Tumors on December 5, 2023
SEATTLE, Nov. 28, 2023 -- Kineta, Inc. (Nasdaq: KA), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology that address cancer immune resistanc ...

Kineta to Present at the 5th Annual Macrophage-Directed Therapies Summit
SEATTLE, Nov. 09, 2023 -- Kineta, Inc. (Nasdaq: KA), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology that address cancer immune resistanc ...

Kineta Unveils Positive New Data from VISTA-101 Clinical Trial of KVA12123 at the Society for Immunotherapy of Cancer’s (SITC) 38th Annual Meeting
Favorable Clinical Safety and Tolerability Profile Observed with No Evidence of CRS-associated Cytokines Achieved Greater Than Dose-Proportional Pharmacokinetics Biomarker Results Demonstrat ...

Kineta Reports Third Quarter 2023 Financial Results and Provides Corporate Update
Announced positive KVA12123 monotherapy safety data from its ongoing phase 1/2 VISTA-101 clinical trial Enrolled the first patient of KVA12123 in combination with pembrolizumab in patients wi ...

Kineta Presents New Preclinical Data on Lead Anti-CD27 Monoclonal Antibody at the Society for Immunotherapy of Cancer’s (SITC) 38th Annual Meeting
Lead Anti-CD27 Monoclonal Antibody Showed High Binding Affinity and Specificity Drives Strong T Cell Activation and Proliferation as well as NK Cell Activation Demonstrated In Vivo Antitumo ...
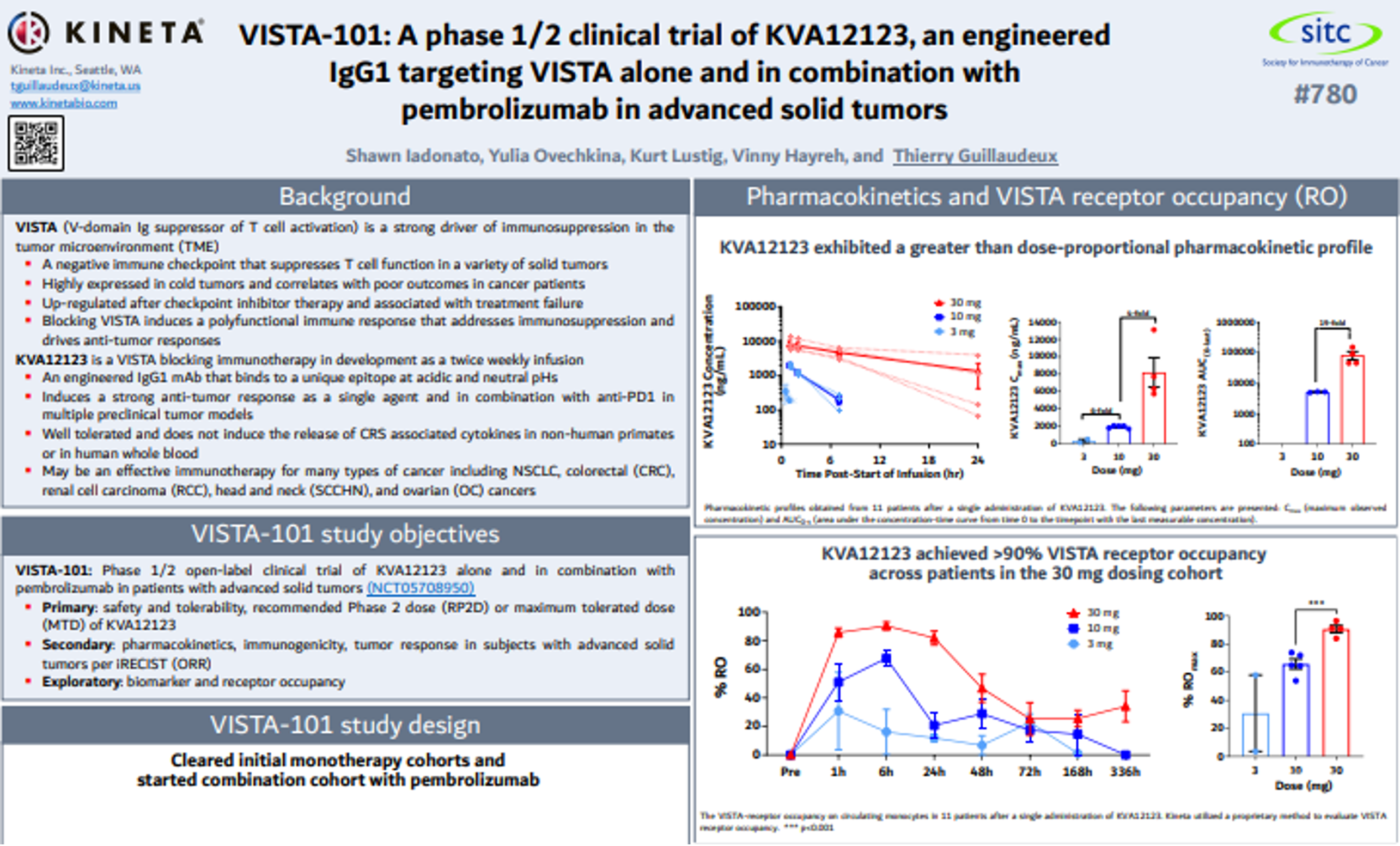
VISTA-101: A phase 1/2 clinical trial of KVA12123, an engineered IgG1 targeting VISTA alone and in combination with pembrolizumab in advanced solid tumors
Thierry Guillaudeux, Phd, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2023 | November 3, 2023

Kineta Announces First Patient Dosed in Phase 1/2 VISTA-101 Clinical Trial of KVA12123 in Combination with KEYTRUDA® (pembrolizumab) in Patients with Advanced Solid Tumors
The Combination Arm (Part B) of the Phase 1/2 Clinical Trial Builds Upon the Initial Safety, Tolerability and Pharmacokinetic Data of KVA12123 in the Monotherapy Arm (Part A) Initial Combina ...

Kineta to Present Preclinical Data on VISTA Blocking KVA12123 at Immuno US 2023
SEATTLE, Oct. 09, 2023 -- Kineta, Inc. (Nasdaq: KA), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology that address cancer immune resistanc ...

Kineta Announces Positive KVA12123 Monotherapy Safety and Biomarker Data from its Ongoing Phase 1/2 VISTA-101 Clinical Trial
Cleared First Three Monotherapy Cohorts with No Dose Limiting Toxicity and No Consistent Pattern of Adverse Events at any Dose Level >90% VISTA Receptor Occupancy Observed in the 30 mg Dos ...
