
Kineta Reports Initial Clinical Response Data at AACR 2024 of its Ongoing Phase 1/2 VISTA-101 Clinical Trial
Partial response and stable disease reported in combination cohort, and durable stable disease observed in monotherapy cohorts Favorable clinical safety and tolerability profile observed with ...

Kineta Reports Full Year 2023 Financial Results and Provides Corporate Update
Partial response and stable disease reported in combination cohort, and durable stable disease observed in monotherapy cohorts in the VISTA-101 trial No dose limiting toxicities observed at a ...

Kineta Provides Update on its Ongoing Phase 1/2 VISTA-101 Clinical Trial of KVA12123 in Patients with Advanced Solid Tumors
Cleared First Four Monotherapy Doses and Initial Combination Cohort with No Dose Limiting Toxicities at any Dose Level KVA12123 Monotherapy Demonstrated Dose Proportional Induction of Pro-inf ...

Kineta Publishes Preclinical Data Demonstrating the Potential of Anti-VISTA Antibody KVA12123 as an Immunomodulatory Therapy for Cancer
VISTA Blocking KVA12123 Monoclonal Antibody (mAb) Engineered to Provide Strong Single Agent Anti-Tumor Activity While Minimizing Cytokine Related Adverse Events KVA12123 recognizes a unique V ...
CD27 is a new promising T-cell co-stimulatory target for cancer immunotherapy
Thierry Guillaudeux, Phd, Chief Scientific Officer Society for Immunotherapy of Cancer (SITC) 2023 | November 3, 2023

Kineta, Inc. Announces Closing of $3 Million Registered Direct Offering Priced At-The-Market under Nasdaq Rules
SEATTLE, Oct. 05, 2023 -- Kineta, Inc. (Nasdaq: KA) (the "Company" or "Kineta"), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology that add ...

Kineta Presents New Preclinical Data on Lead Anti-CD27 Agonist Antibody at AACR Special Conference on Tumor Immunology and Immunotherapy
Preclinical data highlights CD27's mechanism of action and strong anti-tumor activity as a monotherapy and in combination with other checkpoint inhibitors SEATTLE, Oct. 04, 2023 -- Kineta ...

Kineta, Inc. Announces $3 Million Registered Direct Offering Priced At-The-Market under Nasdaq Rules
SEATTLE, Oct. 04, 2023 -- Kineta, Inc. (Nasdaq: KA) (the "Company" or "Kineta"), a clinical-stage biotechnology company focused on the development of novel immunotherapies in oncology that add ...

Kineta to Receive $5 Million Milestone Payment from Merck
Kineta to Receive $5 Million Milestone Payment from Merck Discovery stage milestone triggered by validating an undisclosed target for amyotrophic lateral sclerosis (ALS)SEATTLE, June 29, 2023 -- ( ...
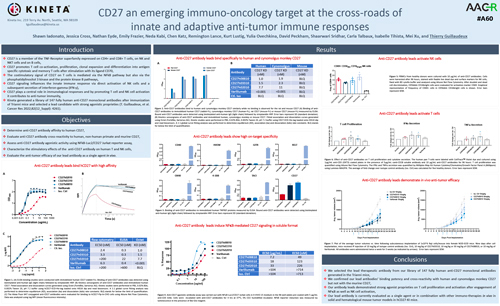
CD27 an emerging immuno-oncology target at the cross-roads of innate and adaptive anti-tumor immune responses
Thierry Guillaudeux, Phd, Chief Scientific Officer American Association for Cancer Research (AACR) Conference on Tumor Immunology and Immunotherapy 2022 | December, 2022
